New Adverse Events are added and are accessible from the Incident issue - either on the Incident's Add or Edit screen. An Adverse Event is always be associated with an Incident, but any single Incident may have multiple Adverse Events. Note that this can be changed through configuration. Simply click on the button Add a New Adverse Event within the Incident screen to add a new adverse event. Information such as the Patient Name, Gender, Age, and Title are automatically carried over from the Incident issue, and appear on the Adverse Event screen. These fields are not able to be altered to give them the same values as their parent Incident. As a minimum, complete any required information and adverse event details such as the Seriousness, Description, Tests, and Outcome and then submit the form.
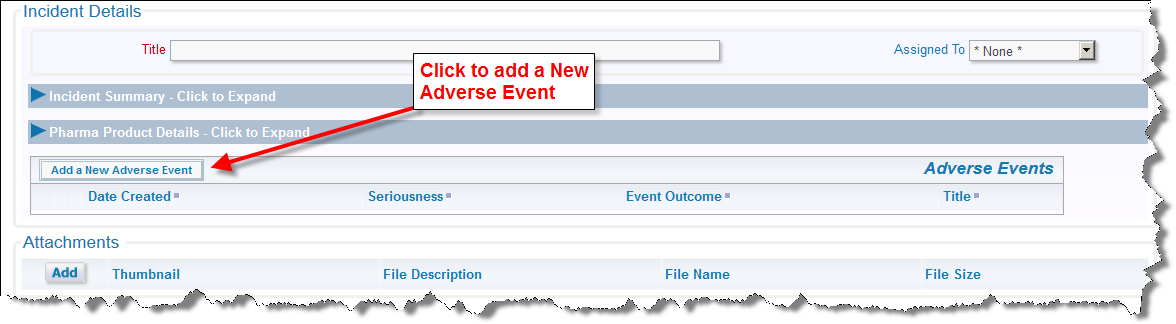
Click to add a new event
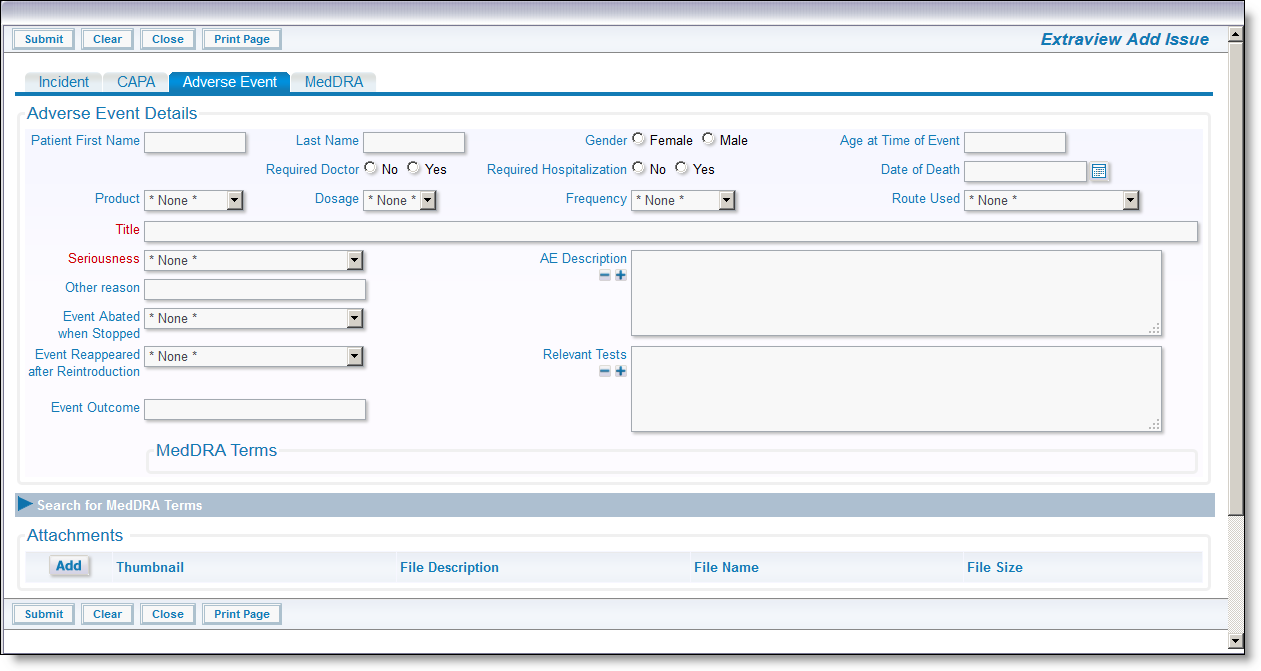
Adverse Event Add Screen
The Seriousness list correlates to the FDA's list of Serious Adverse Events, and is used on any MedWatch form generated by the ExtraView solution.
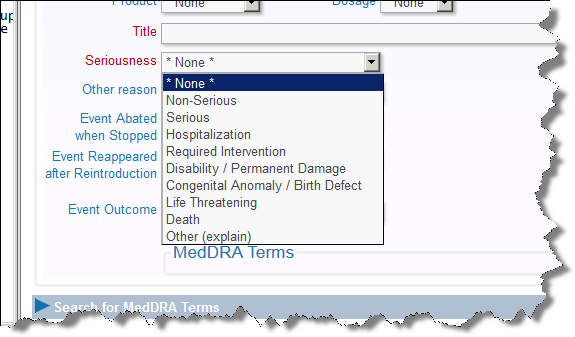
Adverse Event List of Serious Events
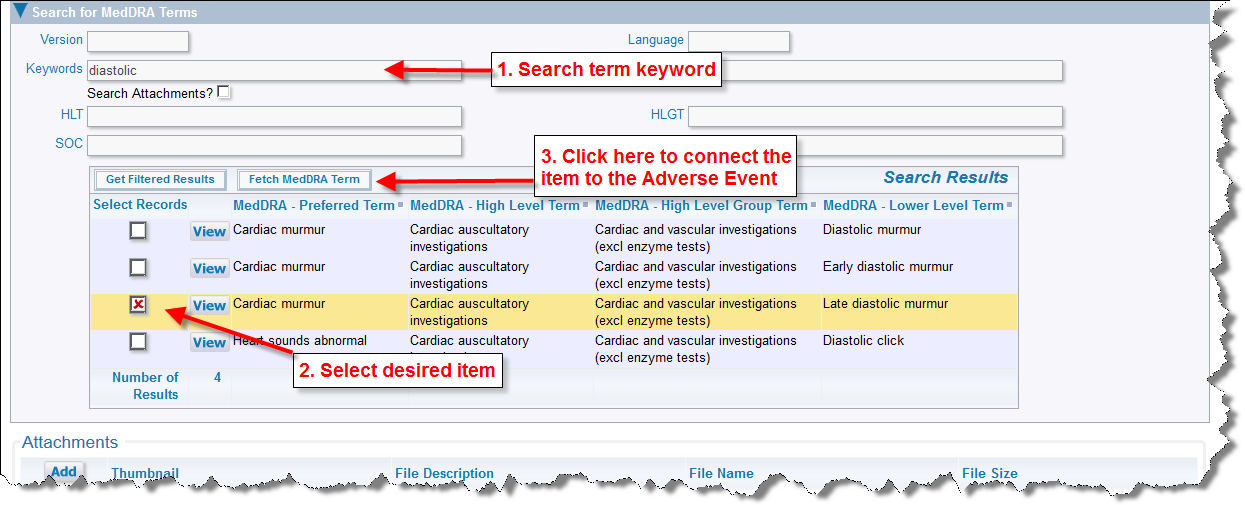
Another key feature of adding Adverse Events is the ability to search for and look up MedDRA dictionary terms. From the Adverse Event Add or Edit screen, expand the folded region entitled Search for MedDRA Terms located towards the bottom of the form. You are presented with a search form where you can enter one or more keywords that are used as filters for the terms for which you are searching. Simply click Get Filtered Results and your results will display.
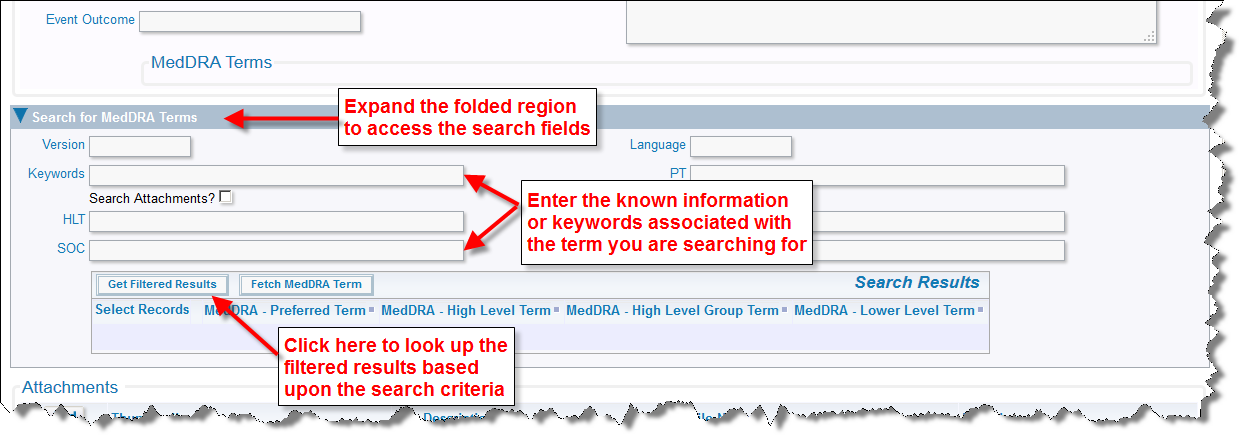
Adverse Event MedDRA Search
Once the term has been selected that you wish to associate with the Adverse Event, click Fetch MedDRA Term. This Adverse Events solution is configured to return a single MedDRA term, but can be reconfigured to relate multiple MedDRA terms per single adverse event.
Adverse Event MedDRA Selection
This is an example of an Adverse Event displaying a related MedDRA term:
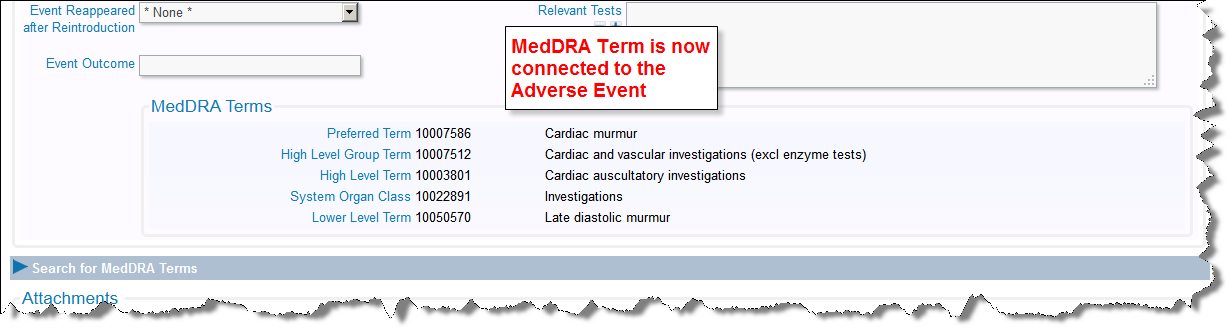
Adverse Event MedDRA Term is connected to the issue
Once the Adverse Event has been submitted, you are returned to the main Incident, where the Adverse Event is now displayed. From there you can View or Edit existing Adverse Event such as the one you have just created, or you may Add additional adverse events to the current incident.
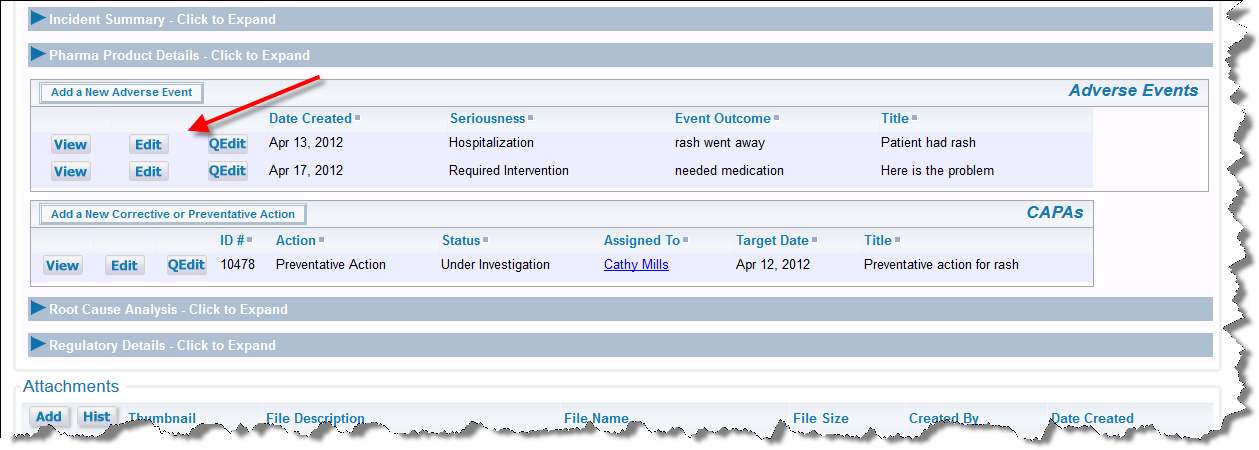
Summary of Multiple Adverse Events for one Incident