This section describes the ExtraView Adverse Events / CAPA solution. This documentation section describes how the basic solution works, how it is implemented within ExtraView and describes how you may alter the implementation for your own needs. Altering the implementation for your own requirements may be done by yourself, or with the assistance of ExtraView Corporation's Professional Services Group. While the solution as provided has all the essential elements of an integrated adverse event reporting / CAPA / quality / root cause analysis / regulatory reporting system, it can be extended to encompass any workflow or process that is envisaged. Indeed, ExtraView has been used to implement adverse events and quality systems at the largest pharmaceutical and medical device manufacturers.
This solution reflects the needs of an organization that interacts with its customers, receiving complaints or incidents, and needing to manage these incidents through an adverse event and CAPA process. The process may result in the need for regulatory reports such as creating MedWatch forms. Input from a MedDRA dictionary can be obtained during the process.
The reader should be familiar with ExtraView concepts, and with ExtraView configuration, as described in the End User Guide and the Administration Guide.
Although the solution covers a broad range of requirements, including CAPA / quality / root cause analysis and regulatory reporting, this documentation collectively describes these as the Adverse Events solution.
Before use, the administrator will provide some simple configuration, to determine whether the solution is being used to manage adverse events for pharmaceutical compounds or for medical devices. It is certainly possible to configure the system to handle both pharma and devices at the same time.
All fields, screens and workflow can be reconfigured as required for your company's needs. The basics of the solution as provided are:
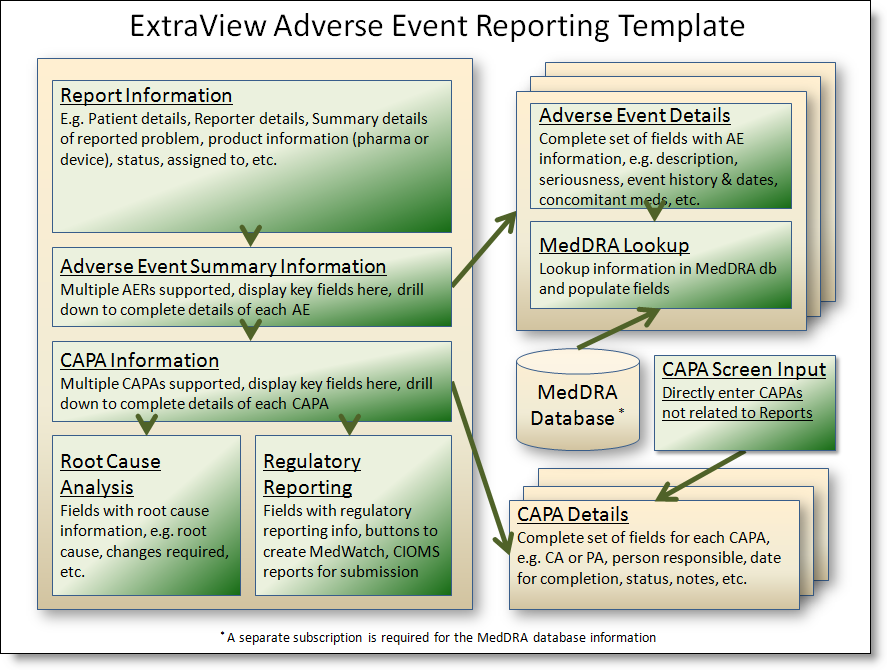
Adverse Events Process