MedDRA - the Medical Dictionary for Regulatory Activities - is a classification of medical terminology for adverse event information. This data is coded to provide a standard set of MedDRA terms, so that health professionals can easily share and analyze data related to the safety of medical products. Users should obtain a copy of the MedDRA dictionary from the appropriate source, as this does not come standard with the ExtraView solution. Please note that the dictionary that is obtained directly from MedDRA is not the correct format to load directly into ExtraView, and therefore will need to be altered. ExtraView support will either provide you with a copy in the correct format, or they will assist you in converting your downloaded version into a more suitable format that is compatible with ExtraView. Having the file in the correct format is also a prerequisite for uploading the latest version of the dictionary.
The MedDRA solution is deliberately configured so that no user can modify the MedDRA terms that have been uploaded. The administrator should not provide permission to any end user to change the data loaded into the template.
It is important to understand that importing a new version of the data does not remove any of the old version of the data. All issues that point to MedDRA dictionary will retain their original link to the specific version of the dictionary that was used when recording the adverse event.
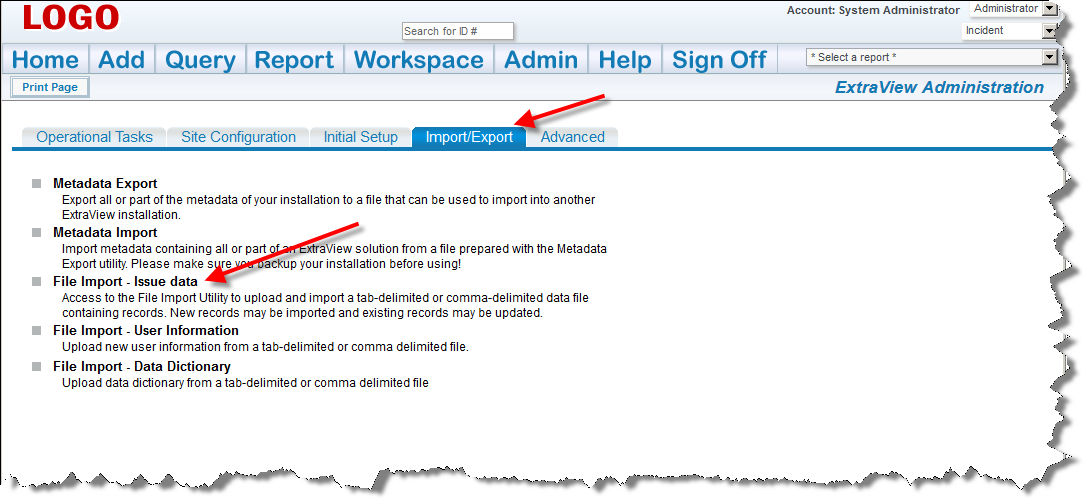
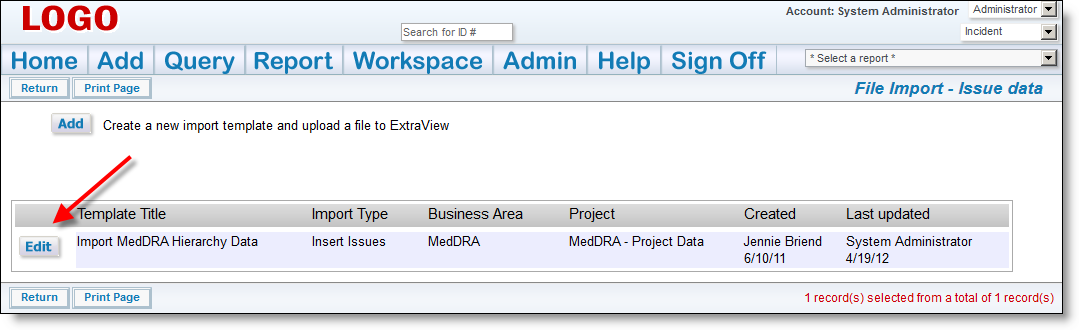
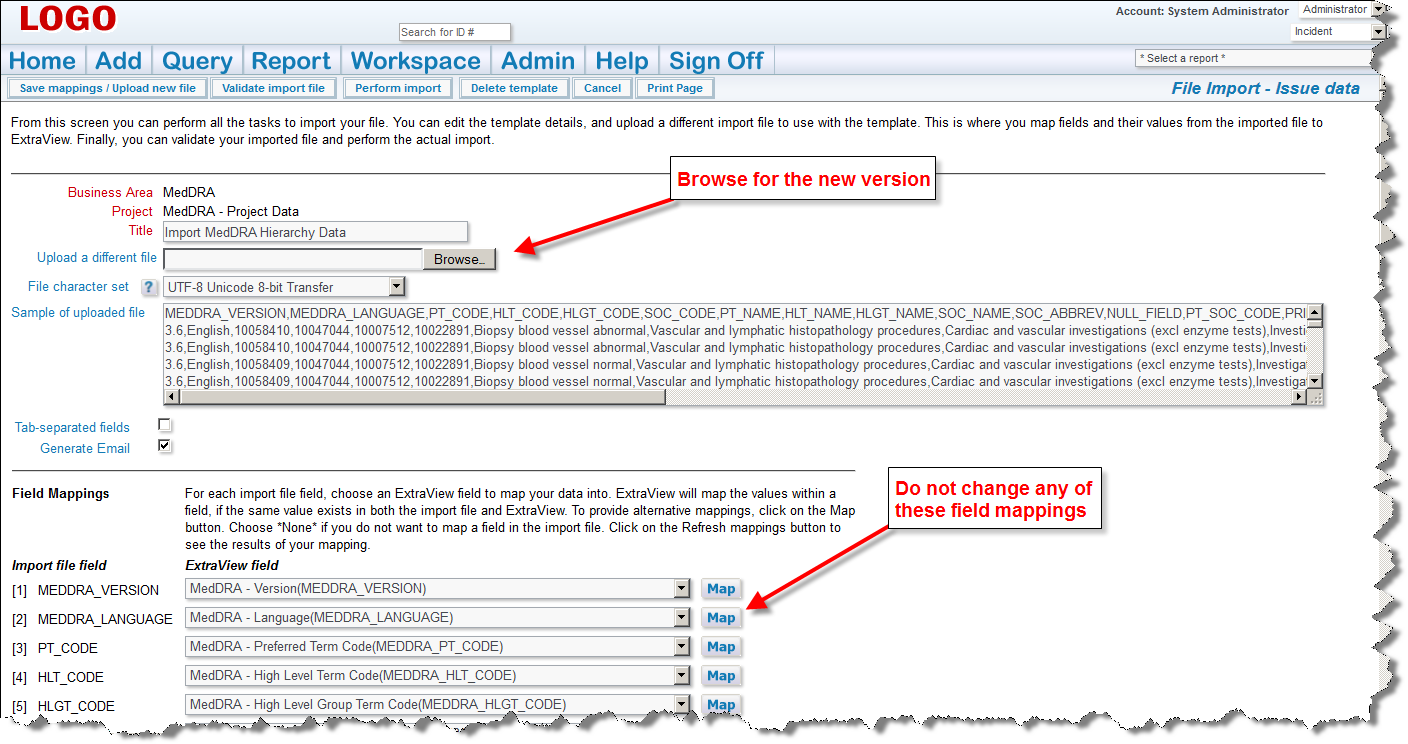
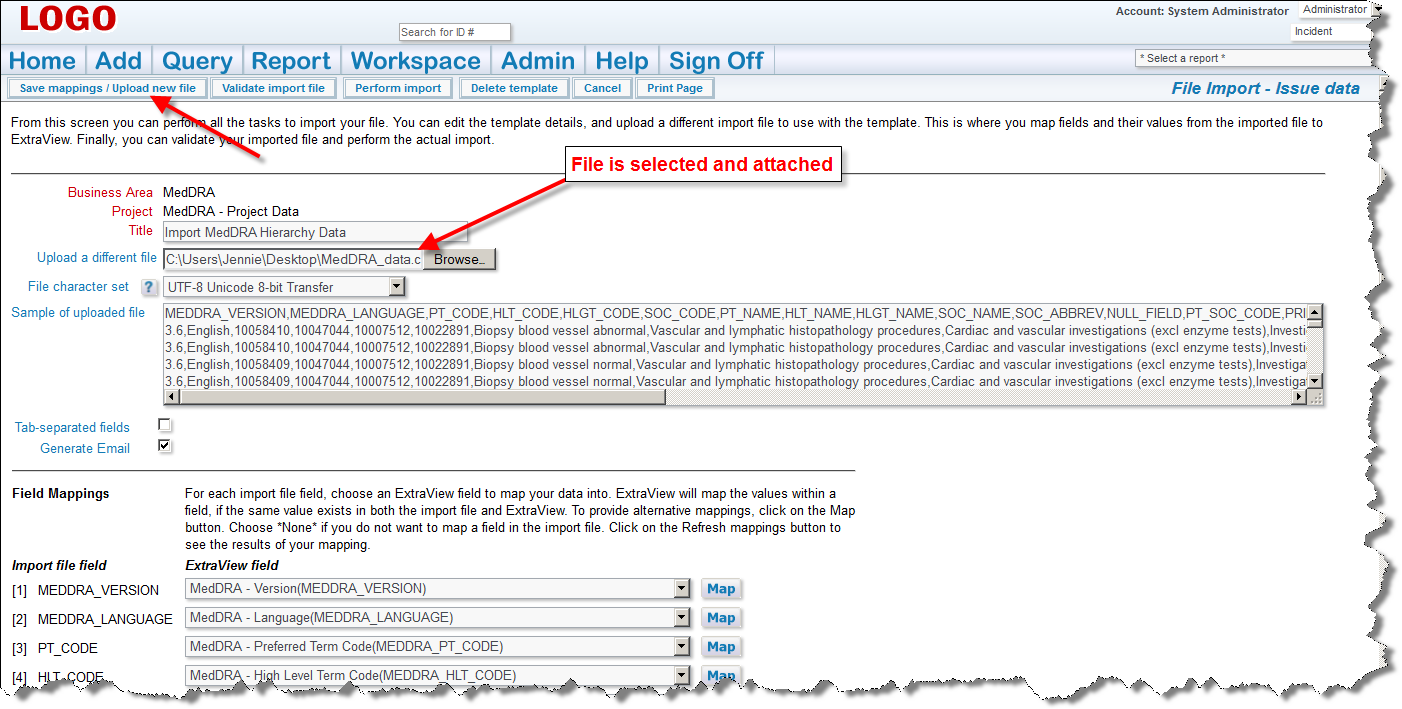
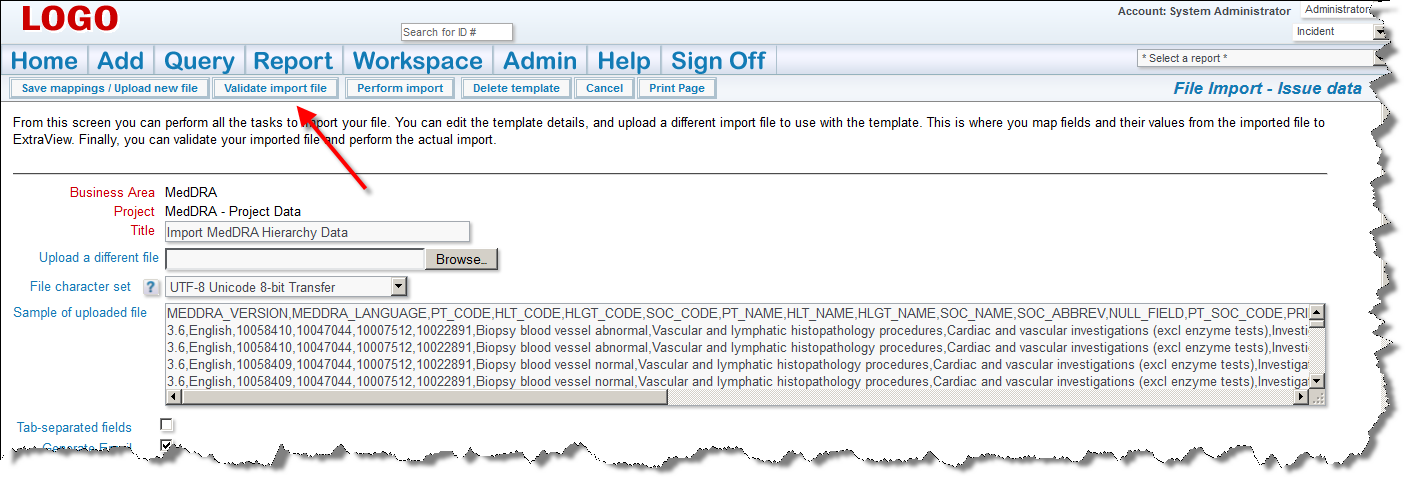
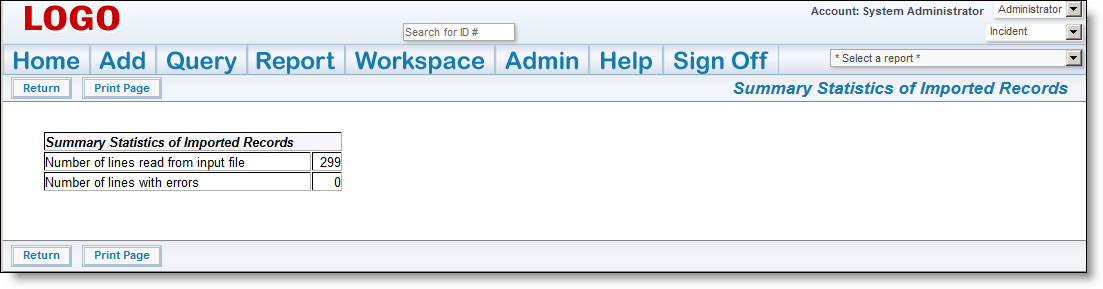
If there are any errors, do not proceed, please contact ExtraView support services
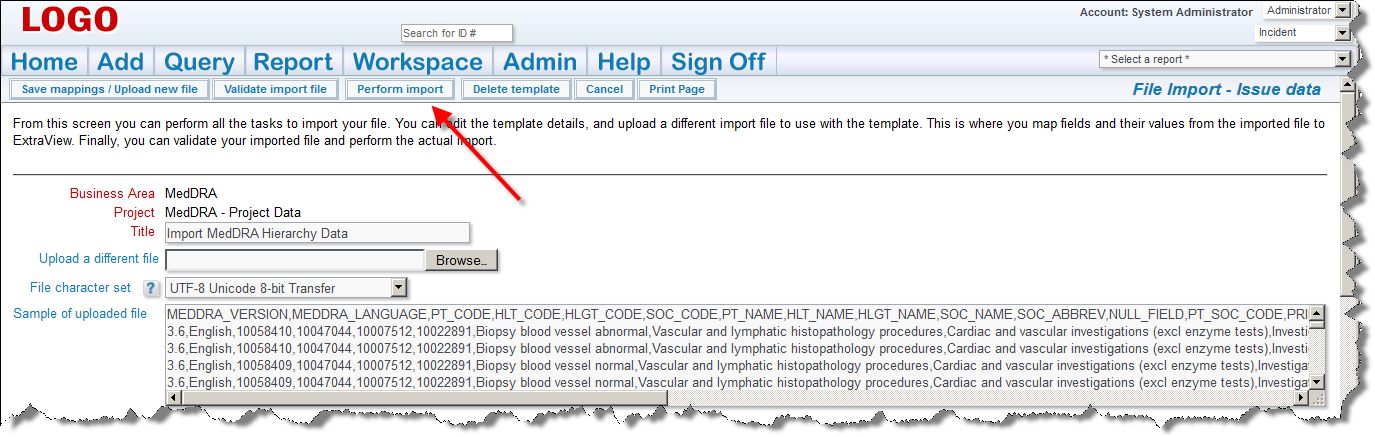
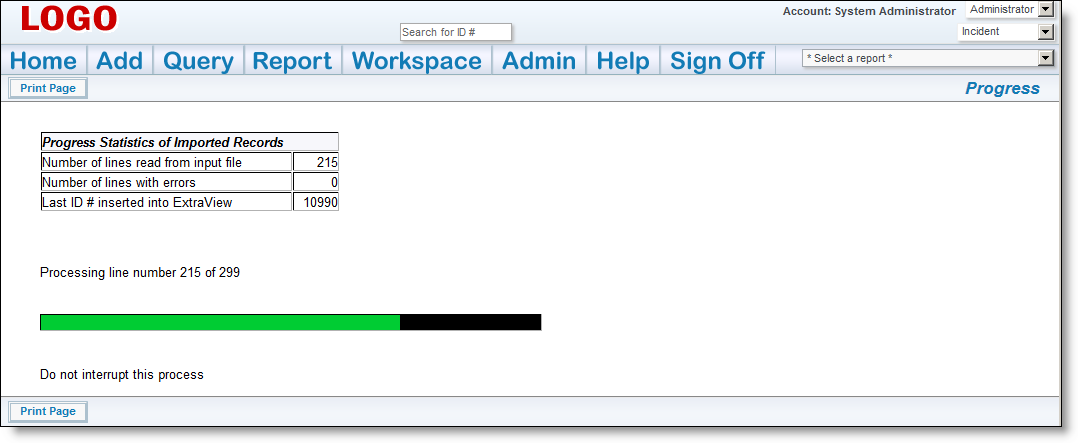
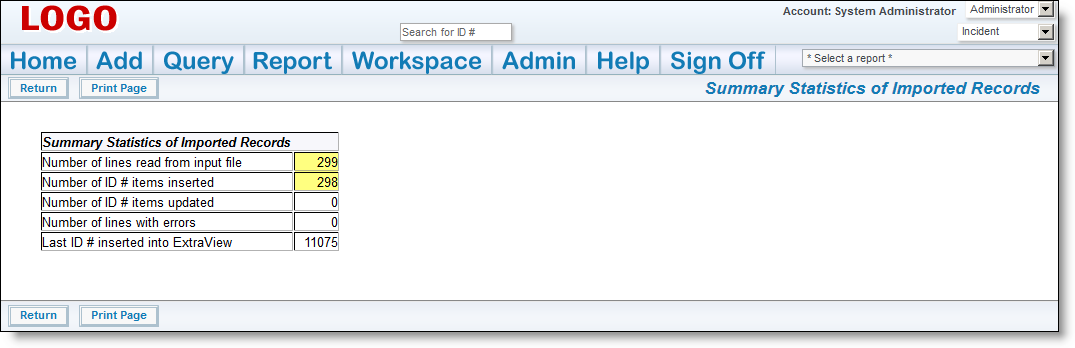
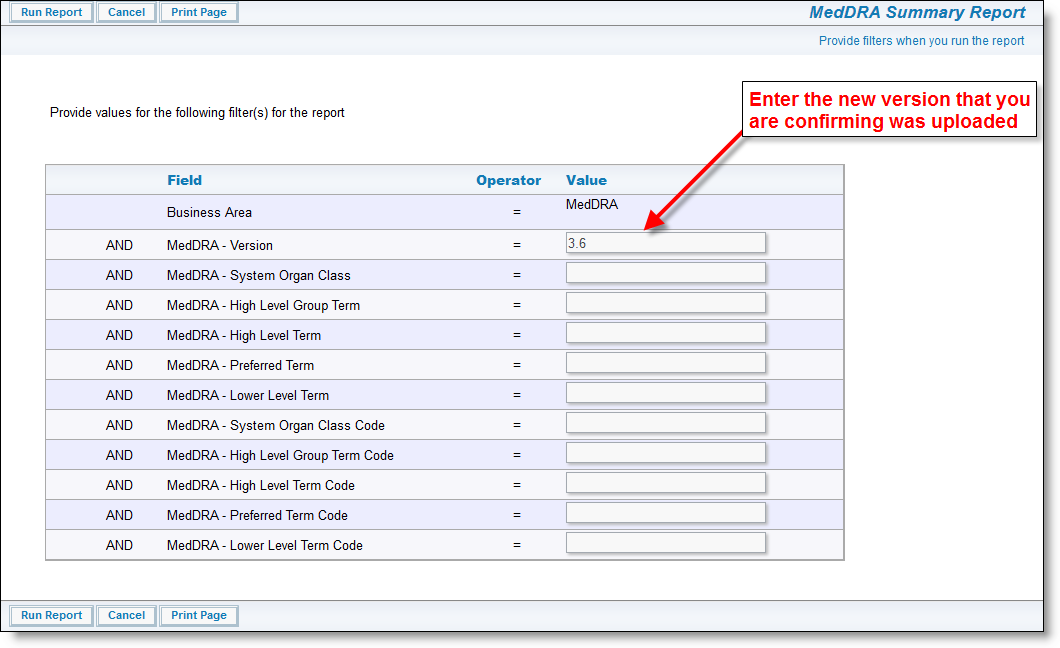
It is imperative that you inform your users that a new version of the MedDRA Dictionary is available. The next time that they add a new incident with a new adverse event all users will need to change the version on the MedDRA term search so that they are looking at the most current set of terms. ExtraView remembers the new entry for subsequent searches, so users will only have to perform this task once, and ExtraView will remember the newest value for the version.
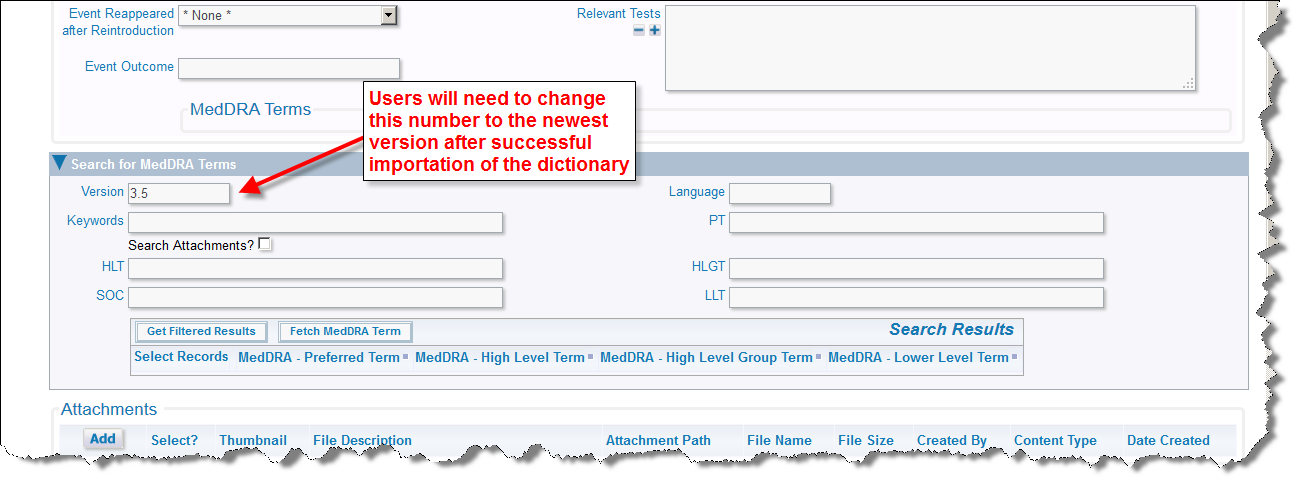
Chance last value of version