The ExtraView Adverse Event / CAPA solution has the ability to generate regulatory for purposes such as MedWatch and CIOMS submissions. Within an Incident screen expand the folded region entitled Regulatory details. Use the prompts that represent a decision tree to determine which, if any, forms should be generated. Click on the Click to Generate Medwatch Form button to generate the FDA MedWatch form, or click on the Click to Generate CIOMS Form. The form is generated using information in the Incident and Adverse Event forms, and appears in the Attachments portion of the Incident screen. You are able to view or print these forms from your browser before submission to the appropriate regulatory body.
Both the MedWatch and CIOMS forms are included in the ExtraView solution. Other regulatory forms can be integrated. Please contact ExtraView support for details.
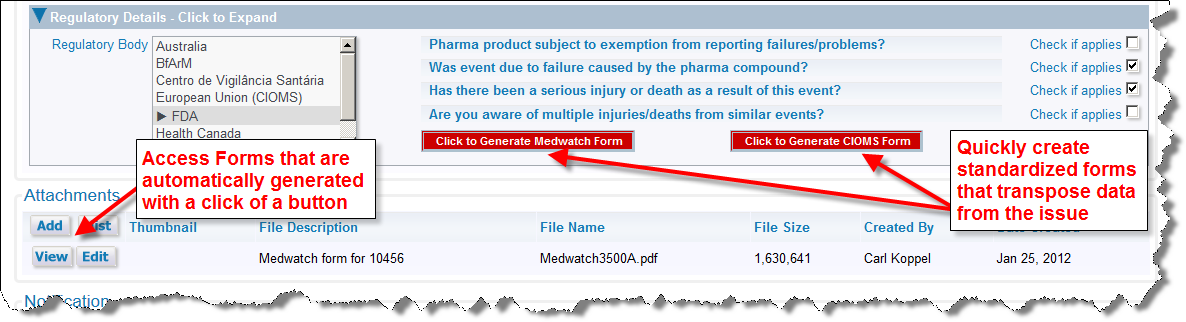
Incident screen with MedWatch form
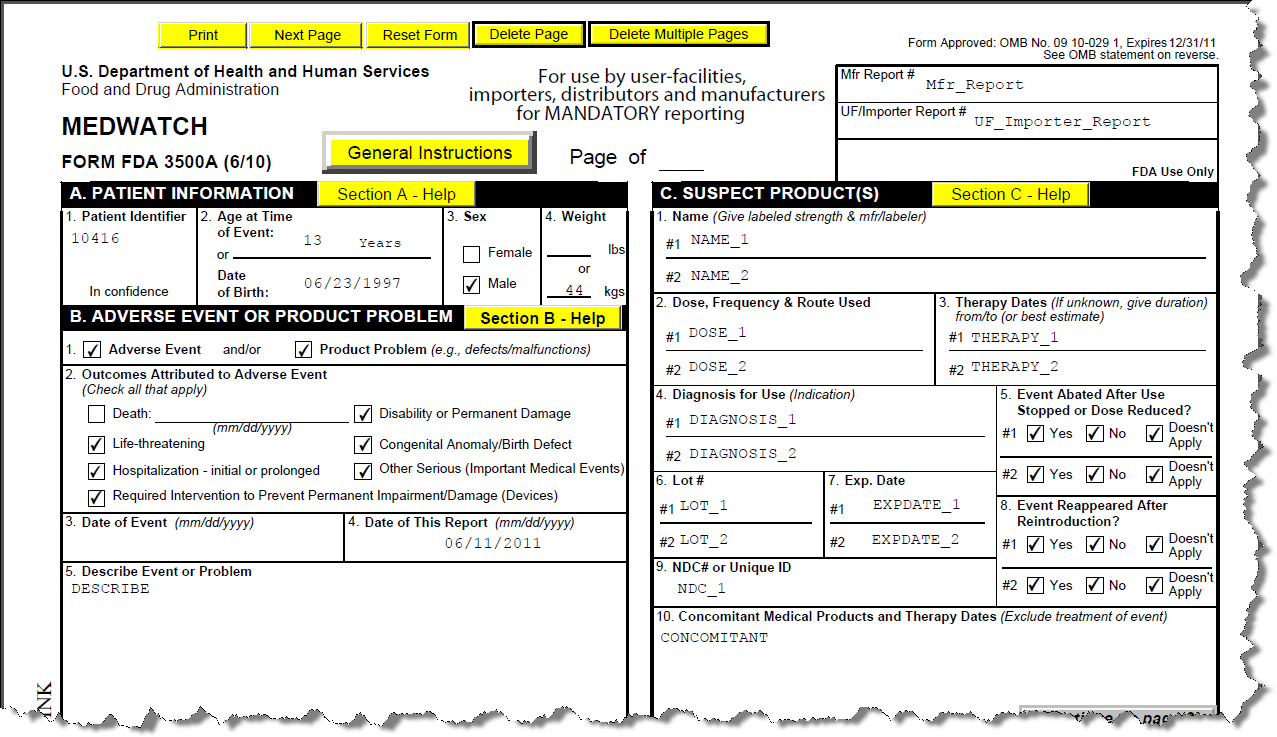
Sample MedWatch Form